Abstract
Objective: CD19 chimeric antigen receptor (CAR) T-cell therapy has changed the standard for treatment of relapsed and/or refractory (r/r) large b-cell lymphoma (LBCL). Currently, pre-treatment evaluation of patients and disease factors do not accurately predict outcomes. This study aims to identify risk factors associated with early mortality prior to day 100 after CAR-T infusion.
Methods: We performed a single-centered retrospective adult patients (pts) with r/r LBCL who received CAR-T cell therapy between 2018-2021. CAR-T cell toxicities graded according to either CAR T-Cell Therapy-Associated Toxicity (CARTOX) grading system prior to May, 2019 or American Society of Transplantation and Cellular Therapy (ASTCT) consensus grading thereafter for cytokine release syndrome (CRS) and immune cell associated neurotoxicity syndrome (ICANS). Comorbidities defined as per Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI). (Sorror et al., 2009). Hepatic dysfunction defined as infection with hepatitis B or C or history of liver cirrhosis or elevated total bilirubin, AST, or ALT on at least two values on two different days from day -24 till day -10 prior to CAR-T. Cardiovascular (CV) comorbidity determined by presence of coronary heart disease, congestive heart failure or reduced ejection fraction detected by echocardiogram. Psychiatric disturbance defined as presence of depression and/or anxiety requiring treatment or consultation. Patient demographics, disease characteristics and clinical outcomes summarized through descriptive statistics. Wilcoxon rank sum test used to evaluate difference in a continuous variable between patient groups. Kaplan-Meier method used to estimate time-to-event endpoints including progression free survival (PFS), and overall survival (OS).
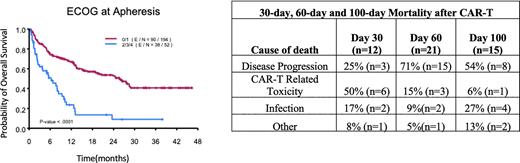
Results: Two hundred and fifty patients included in analysis. Median age of 61years (range: 18-89) and 30.8% of patients were female (n=77). Histology was categorized as LBCL (65.9%) and included diffuse LBCL or high grade LBCL, transformed follicular lymphoma (26.9%), primary mediastinal b-cell lymphoma (6.4%) or follicular lymphoma (0.8%). Majority of pts received axicabtagene ciloleucel 95.6% (n=239) while 4.4% (n=11) received tisagenlecleucel and 52% (n=130) of pts received bridging therapy. CRS of any grade was 90.4% ; ICANS any grade was 57.2% while grade 3 CRS/ICANS occurred 6.4% and 38.4% respectively. Median follow-up 24.7 months (mo) (95% CI: 20.7 ~ 26.4 mo). One-hundred twenty-eight pts died during follow-up, 48 pts died within 100 days, and most common cause of death was progression of lymphoma (29.2%; n=73). Early 30-day mortality occurred in 4.8% pts and causes of death due to CAR-T related toxicity in 6 pts, disease progression in 3 pts, and infection in 1 pts. 100-day mortality rate was 24.6% (n=32) for pts >60 years old vs 13.3% (n=16) for <60 (p-value=0.02). 100-day mortality rate was 36.5% for ECOG 2-4 vs 14.9% for ECOG 0-1 (p-value=0.0005). In terms of non-relapsed mortality (NRM), risk stratification according to cumulative HCT-CI score was not associated with higher 100-day NRM (p=0.24). Pts with cardiac score of 1 had a higher NRM (26.9% vs. 8.1%; p-value=0.005). The 100-day NRM rates for hepatic score of 0, 1, and 3 were 8.9%, 14.3%, and 50% (p-value=0.04). Pts with psychiatric disturbance score of 1 had higher 100-day NRM (19.6% vs. 7.8%; p-value=0.03).
Conclusion: This single center retrospective study observed an increase in 100-day mortality in correlation with pre-treatment performance status, and older age. Pts with cardiac comorbidity, hepatic dysfunction or psychiatric disturbance had a higher 100-day NRM. Similar to other studies, the combined HCT-CI score was not predictive of NRM, but individual variables were associated with higher non-CART related toxicity. These findings require validation with further studies.
Disclosures
Strati:ALX Oncology: Research Funding; Astrazeneca Acerta: Research Funding; Kite Gilead: Consultancy; TG Therapeutics: Consultancy; ADC Therapeutics: Consultancy, Research Funding; Hutchinson MediPharma: Consultancy; Roche Genentech: Consultancy; Sobi: Research Funding. Steiner:BMS: Research Funding; Seagen: Research Funding; GSK: Research Funding; Rafael Pharmaceuticals: Research Funding. Nair:Incyte Corporation: Honoraria. Flowers:EMD: Research Funding; Takeda: Research Funding; Ziopharm: Research Funding; TG Therapeutics: Research Funding; Sanofi: Research Funding; V Foundation, Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research: Research Funding; Morphosys: Research Funding; Pfizer: Research Funding; National Cancer Institute: Research Funding; Genmab: Consultancy; Spectrum: Consultancy; Pharmacyclics: Research Funding; Karyopharm: Consultancy; Eastern Cooperative Oncology Group: Research Funding; Cellectis: Research Funding; Amgen: Research Funding; Janssen Pharmaceutical: Research Funding; Kite: Research Funding; Gilead: Consultancy, Research Funding; SeaGen: Consultancy; Iovance: Research Funding; Pharmacyclics/Janssen: Consultancy; Allogene: Research Funding; Adaptimmune: Research Funding; Acerta: Research Funding; Genentech/Roche: Consultancy, Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Denovo Biopharma: Consultancy; Xencor: Research Funding; Burroughs Wellcome Fund: Research Funding; Guardant: Research Funding; NPower: Current holder of stock options in a privately-held company; 4D: Research Funding; Celgene: Consultancy, Research Funding; BeiGene: Consultancy; Abbvie: Consultancy, Research Funding; Bayer: Consultancy, Research Funding. Srour:Orca Bio: Research Funding. Champlin:General Oncology: Other: Data Safety Monitoring Board; Actinium: Consultancy; Cell Source Inc.: Research Funding; Omeros: Consultancy; Kadmon: Consultancy; Bluebird: Other: Data Safety Monitoring Board; Johnson &Johnson: Consultancy. Kebriaei:Pfizer: Consultancy; Jazz: Consultancy; Kite: Consultancy; Amgen: Research Funding; Ziopharm: Research Funding. Nastoupil:ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria; Genentech/Roche, MEI, Takeda: Other: DSMC; BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding. Mistry:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees. Shpall:Fibroblasts and FibroBiologics: Consultancy; NY blood center: Consultancy; Navan: Consultancy; axio: Consultancy; adaptimmune: Consultancy; Bayer: Honoraria; Takeda: Patents & Royalties; Affimed: Other: License agreement. Nieto:Affimed: Other: Scientific advisory Board, Research Funding; Secura Bio: Research Funding; Astra Zeneca: Research Funding. Westin:Abbvie/GenMab: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; MorphoSys/Incyte Corporation: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; MonteRosa: Consultancy; Calithera: Consultancy, Research Funding; Iksuda: Consultancy; Merck: Consultancy; Novartis: Consultancy, Research Funding; SeaGen: Consultancy. Neelapu:Bluebird Bio: Consultancy, Honoraria; Unum Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Calibr: Consultancy, Honoraria, Other: Personal fees; Adicet Bio: Consultancy, Honoraria, Other: Personal fees, Research Funding; Legend Biotech: Consultancy, Honoraria, Other: Personal fees; Precision Biosciences: Consultancy, Honoraria, Other: Personal fees, Research Funding; Incyte: Consultancy, Honoraria, Other: Personal fees; Cell Medica/Kuur: Consultancy, Honoraria, Other: Personal fees; Allogene Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Pfizer: Consultancy, Honoraria, Other: Personal fees; Celgene: Consultancy, Honoraria, Other: Personal fees, Research Funding; Novartis: Consultancy, Honoraria, Other: Personal fees; Bristol Myers Squibb: Consultancy, Honoraria, Other: Personal fees, Research Funding; Merck: Consultancy, Honoraria, Other: Personal fees, Research Funding; Kite: Consultancy, Honoraria, Other: Personal fees, Research Funding; Medscape: Consultancy, Honoraria; Aptitude Health: Consultancy, Research Funding; Bio Ascend: Consultancy, Honoraria; Poseida: Research Funding; Cellectis: Research Funding; Karus Therapeutics: Research Funding; Acerta: Research Funding; Takeda Pharmaceuticals: Patents & Royalties: related to cell therapy.. Ahmed:Servier: Membership on an entity's Board of Directors or advisory committees; Myeloid Therapeutics: Consultancy; Merck: Research Funding; Chimagen: Consultancy, Research Funding; Xencor: Research Funding; Tessa Therapeutics: Consultancy, Research Funding; Seagen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal